September 22, 2021
Schlafender Hase Team
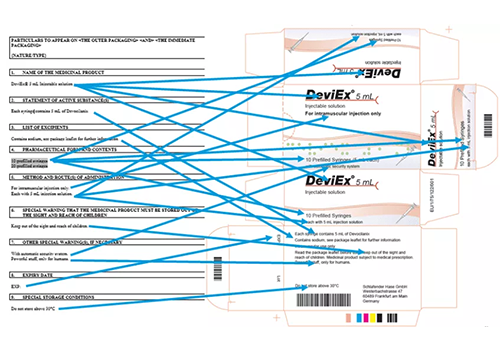
MDR UDI and Device Registration
The new European Database on Medical Devices, known as EUDAMED, was originally scheduled to launch in May 2020,
- September 22, 2021
- By: Marc Chaillou
2 minutes read
MDR UDI and Device Registration
The new European Database on Medical Devices, known as EUDAMED, was originally scheduled to launch in May 2020,