With electronic labeling (e-labeling) gaining momentum across healthcare markets, are you ready to maximize the benefits?
The healthcare sector has been slower than consumer industries in adopting electronic labeling (e-labeling). Asian countries and Australia have been among those at the forefront of the movement, but the adoption of e-labeling has now gained momentum in European countries.
Many countries are currently focused on promoting electronic alternatives to the traditional physical inserts with small print that have been commonly used with medicines and medical devices for a long time. These electronic alternatives aim to make information more accessible and user-friendly.
In Europe, the European Medicines Agency (EMA) is piloting e-labeling for prescription drugs in parallel with physical labeling.[1] Meanwhile, the US Food and Drug Administration (FDA) DailyMed searchable database, which was established in 2006 with just one electronic label, today has over 140,000 labeling documents for those products within the brief of the FDA [2]. In 2003 the FDA published its electronic labeling rule stipulating that labeling content “must be submitted electronically in a form that FDA can process, review, and archive.” [3]
While for some companies and regions, e-labeling is a looming reality, for others it has long been standard practice, demanding its own integrated workflow (more about that later).
What is e-labeling?
In its broadest sense, e-labeling is product labeling information online, most commonly accessed by the patient, healthcare professional or consumer by scanning a barcode (e.g. QR code) with a mobile device. The information may be available in different formats, such as audio or video, as well as in written form. Specific information received can be easily customized and vary according to the geographical location of the recipient.
At present in the healthcare sector, e-labeling such as paper-based patient information leaflets (PILs) and instructions for use (IFUs) complements physical labeling rather than replaces it. This duality in particular – a hybrid approach – presents its own challenges and demands a carefully integrated approach.
Labeling from another age
Virtually all physical labeling today has a digital correlative, or at least a legacy digital copy (e.g. an approved original, such as a Word document or PDF). Therefore, it might be difficult to imagine the pre-desktop publishing era of labeling when inserts were produced initially in hand-written drafts before being manually typed up in many different versions (perhaps by a professional typist), proofread and corrected manually, approved in numerous cycles, typeset, printed, packaged and distributed to the patient or consumer in a physical format.
Although it may never be phased out entirely, physical labeling remains a child and necessity from this pre-digital, pre-internet age. And some pre-digital processes, like manually proofreading digital documents, are still present in many companies’ labeling workflows today. Others have long fallen by the wayside.
Today, a regulatory affairs professional will work from the very beginning of the workflow with digital document, generally in XML and MS Word.
Challenges of physical labeling
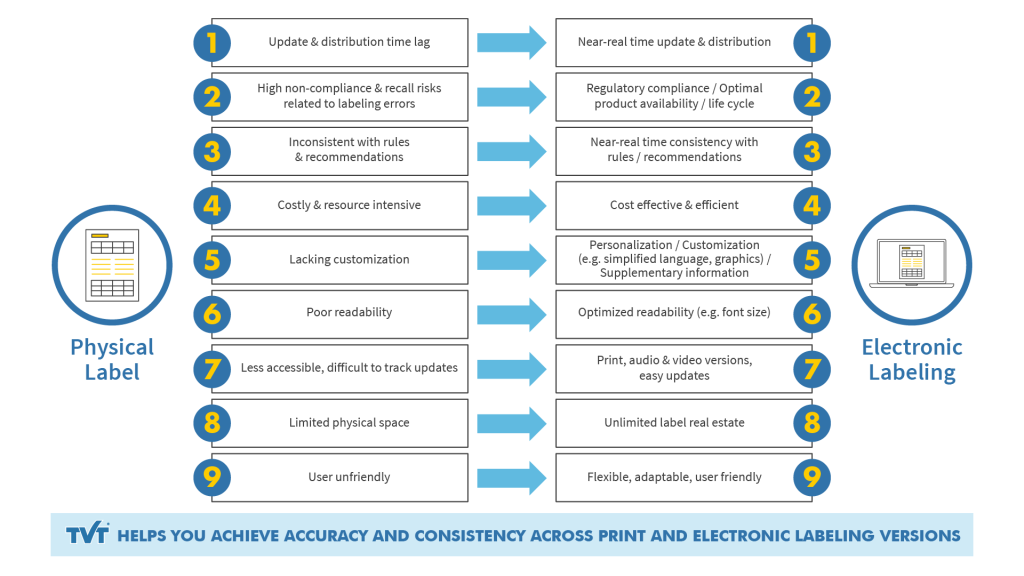
For patients (and in most cases healthcare professionals), the major disadvantages of physical labeling stem from the limited labeling space, which in turn places limits on print size and the amount of information that can be fit onto labeling, and even the type of language used (extreme brevity, sometimes resulting in dense, user-unfriendly language).
Physical labeling is also prone to delays in updating information, because the process of printing and distribution is longer and invariably involves more participants and steps. As a result, patients may not receive the latest information as quickly as they could or they should.
To a greater or lesser degree, maintaining consistency and accuracy using just physical, manual means is inefficient, time-consuming and nerve-wracking. Information updates are slow and difficult to manage and track. The risk of a worst-case-scenario such as damage to patient health and product recall is much higher due to inconsistency (non-compliance) with latest guidelines. As we will see, these disadvantages can become amplified in the hybrid physical-digital approach and present workflow challenges.
Benefits of e-labeling
Because virtually all labeling today begins with a digital document such as XML, MS Word or a PDF, from a practical perspective, providing an e-labeling version of hardcopy labeling is in theory a simple task of offering it online.
Real-time updates
Electronic versions can be easily updated online in near-real time
Greater consistency with current regulations
Ability to implement updates faster means e-labeling will reflect current regulations faster and more accurately across different markets
Cost-effective
Physical labeling is less cost-effective than digital formats
Reduced environmental impact
Paperless formats save natural resources
New opportunities for personalization
E-labeling allows patients and healthcare professionals to access information tailored to their specific needs beyond the requisite labeling information
Additional product information can be provided
Required labeling information for all patients or healthcare professionals can be complemented by further information about the product, its use and its benefits
Better readability and accessibility
Online text can be magnified or read aloud for those with poor sight
Improved user-friendliness
Such as additional simplified language versions or simplified layouts
Implications for regulatory affairs professionals and workflows
However, the introduction and use of e-labeling to augment physical labeling raises the question of how to best manage proofreading workflows for the creation, submission and updating of both physical and online formats to ensure ongoing compliance with regulations.
In terms of proofreading, the biggest challenge is one of online proofreading efficiency while also maintaining consistency between format versions. Recognizing this, Schlafender Hase has integrated into TVT version 12 a functionality allowing the user to simply insert a website URL and compare the online text or document with the approved master document. The online version is automatically loaded into TVT and it can then be proofread as it would be if in any other format. TVT 12 can also compare files in their native HTML and XML formats.
The uses and benefits are many. For example, this process is useful not only for comparing approved original documents with digital content, but also for comparing current physical versions of labeling in a digital format with the current online version in order to find inconsistencies
While we can expect physical labeling to continue to be a feature of medicines and medical devices in the foreseeable future, e-labeling offers many benefits and takes us one step further towards ensuring patients and healthcare professionals receive the most accurate and up-to-date information upon which they can make informed decisions.
Would you like to find out how to best integrate your proofreading workflows in an e-labeling environment? Contact us and we can help you.