In December 2022 the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) was enacted in the US. It marks a major change in the regulation and oversight of cosmetics marketing and cosmetics manufacturing facilities. What changes will MoCRA bring? Who does it affect, and how can you meet the challenges and remain MoCRA compliant?
Preparing for MoCRA
What is MoCRA and when does it come into force?
The US Food and Drug Administration (FDA) describes the new MoCRA cosmetics regulations as “the most significant expansion of FDA’s authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic (FD&C) Act was passed in 1938.” [1]
It is designed to increase and ensure the safety of cosmetic products for consumers. FDA MoCRA cosmetic regulations cover a wide range of personal care and beauty products, with some exceptions explicitly laid down by the FDA, such as for products within the scope of the FDA’s drug and medical device regulations.
Modernization of Cosmetics Regulation Act of 2022 (MoCRA) comes into force December 29, 2023, although cGMP provisions will take longer, and facility registration and product listing requirements have been pushed back six months.
Key MoCRA cosmetic regulations changes:
- Registration of cosmetic facilities
- Product listing (including ingredients)
- Safety Substantiation and Adverse event reporting
- Documentation (FDA has new access and review powers)
- cGMP requirements
- Safety substantiation and FDA recall powers
- Labeling changes (i.e. fragrance allergens and other declarations)
Registration of Cosmetic Manufacturing Facilities
Those affected by MoCRA had until December 29, 2023 to register their facilities, and new facilities after that date had to be registered within 60 days of products being marketed. This has since been moved back to July 1, 2024. The FDA can suspend facility registration if there is a “reasonable probability of causing serious adverse health consequences or death to humans”, and there is reasonable belief that other products in the facility may be similarly affected.[2]
The FDA is developing an electronic submission portal (Cosmetics Direct) online to streamline the process of submitting facility registration and product listing, complementing paper-based forms. [3]
Product Listing
Originally, all products had to be listed by December 29, 2023 by the “responsible person”, or within 120 days after interstate marketing begins for new products released after this date. As with facility registration, this was extended to July 1, 2024.
A responsible person is “the manufacturer, packer, or distributor of a cosmetic product whose name appears on the label of such cosmetic product in accordance with section 609(a) of the FD&C Act or section 4(a) of the Fair Packaging and Labeling Act.” [4]
Safety Substanitation and Adverse Event Reporting/Record Keeping
Although the FDA does not approve cosmetics prior to market release or have a list of tests prior to demonstrate the safety of cosmetic products or ingredients, responsible persons must maintain records “supporting adequate safety substantiation”, based on robust scientific methods. [5] Standards of record-keeping are therefore higher, and in most cases that means more and better-quality documentation is required than used to be the case.
The responsible person must report to the FDA any serious adverse events within 15 business days. The FDA has powers to access documents during inspections. Furthermore, regulations give the FDA the authority to order a mandatory recall of a product if the responsible person refuses to recall its product and the FDA believes “a cosmetic is adulterated or misbranded and the use of or exposure to the cosmetic will cause serious adverse health consequences or death.” [6]
Cosmetic Product Labels and cGMP
Cosmetics are governed by Good Manufacturing Practices (FDCA Section 606). The FDA has committed to publishing a notice of proposed rulemaking not later than December 29, 2024, and a final rule by December 29, 2025. Regulations give the FDA expanded powers of inspection to ensure compliance.
cGMP and registration and listing rule exemptions apply to small businesses with average gross annual sales of less than $1 m over the previous three years, but certain high-risk cosmetics products are not subject to exemptions. [7]
“Exemptions do not apply to facilities that manufacture or process, or responsible persons for, the following cosmetic products:
- Products that regularly come into contact with mucus membrane of the eye under customary or usual conditions of use.
- Products that are injected.
- Products that are intended for internal use.
- Products that are intended to alter appearance for more than 24 hours under customary or usual conditions of use and removal by the consumer are not part of such conditions of use.” [8]
In terms of cGMP, the FDA has indicated that it is moving in the direction of ISO 22716:2007 (Cosmetics Good Manufacturing Practices), which is currently accepted in Europe. [9]
Cosmetics intended for use by professionals must be labeled with a statement to this effect, new responsible person” contact details apply, and fragrance allergens must now be disclosed on product labeling. Disclosure of fragrance allergens comes into effect on June 29, 2024.
How You Can Keep Ahead of the MoCRA Regulations with TVT
MoCRA brings new challenges for the cosmetics sector in terms of compliance. The largest of these challenges will be document management, ensuring the accuracy of documents, and updating documents – especially labeling. Many companies will need to update workflows, and upgrade workflow management systems and information systems. It will also place greater strain on available staff resources, which in turn will require new efficiencies in order to minimize the costs. TVT comparison software will help you achieve these efficiencies and remain MoCRA-compliant.
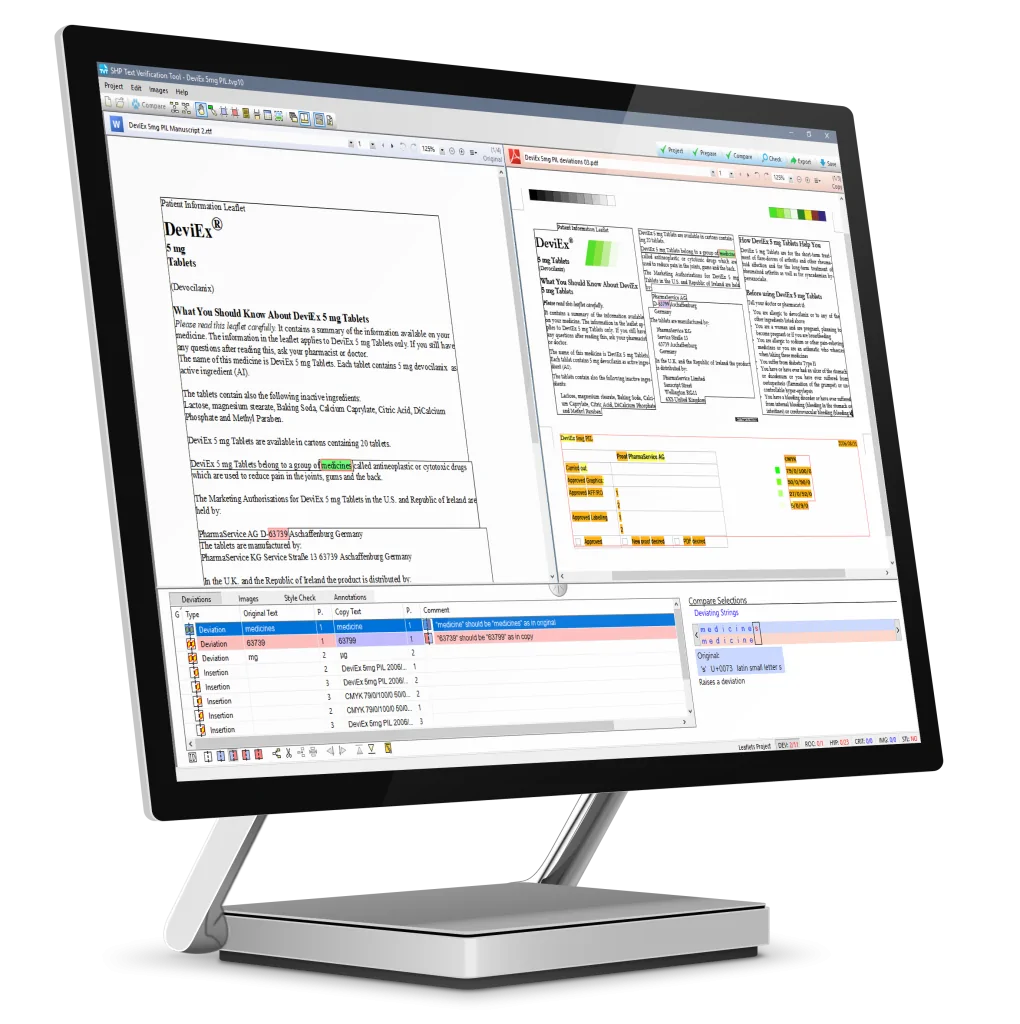
How TVT works
TVT comparison software allows you to quickly and easily compare your approved documents in any format and language with another document version (e.g. a PDF printer proof of labeling). Any differences between the documents (errors) are immediately highlighted. Documents can be annotated for change, and all actions are captured using printing and report functions. TVT is intuitive, saves time, minimizes the risk of error, while increasing the accuracy of documents.
Furthermore, TVT can be integrated with your current information management systems. With some of the requirements of MoCRA (e.g. adverse event reporting and recalls) leaning heavily on regulations in the pharmaceuticals industry, you also benefit from the regulatory experience of Schlafender Hase in this sector.
If you are working with graphics, barcodes and complex terminologies, TVT Artwork®, TVT Barcode® and TVT Spelling® are add-on modules for verifying these elements.
Furthermore, TVT can be used to compare documents with website content by simply inserting the website URL. This helps you align your online documentation with approved text.
How other companies are already benefiting from using TVT:
– Compliance
Reduces errors and omissions in labelling, packaging and regulatory documentation. Minimizes the risk of costly litigation resulting from error and non-compliance.
– TVT Reduces risk of recalls
Serious adverse events may even result in the FDA enforcing a product recall if you do not do this voluntarily. Safety is compromised, market share suffers, and so too does your reputation for marketing safe cosmetic products.
– TVT helps you comply with structured product labelling (SPL)
SPL is an FDA standard for formatting product and drug information. It is necessary to facilitate the easy exchange of data in machine-readable format. Facility registration and product listings required under MoCRA are included within this SPL framework. [10]
– Your documentation will be clean and consistent
Whenever the FDA demands access or you provide documentation to the FDA. Inaccurate and “dirty documentation” jeopardizes cosmetic product sales, delays launch and increases cost. You are better-positioned to meet the higher demands placed on documentation (e.g. serious adverse events).
Contact us and, based on over 20 years’ experience in regulated industries, we can help you master the challenges of MoCRA.
- www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra
- https://www.khlaw.com/insights/cosmetics-regulations-get-makeover-congress-enacts-new-requirements-under-mocra?language_content_entity=en
- https://www.fda.gov/media/173606/download?attachment
- https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra
- https://www.fda.gov/cosmetics/cosmetics-science-research/product-testing-cosmetics
- https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra
- https://frostbrowntodd.com/modernization-of-cosmetics-act-of-2022-overhauls-fda-regulation-of-the-cosmetics-industry/
- https://www.fda.gov/cosmetics/cosmetics-news-events/fda-issues-draft-guidance-registration-and-listing-cosmetic-product-facilities-and-products
- https://www.fda.gov/media/169317/download?attachment
- https://www.fda.gov/cosmetics/cosmetics-news-events/fda-publishes-structured-product-labeling-spl-implementation-guide-validation-procedures-cosmetic





